- Home
- Blog
- Medical Dermatology
- New relief for the intractable itch of chronic hives, or chronic spontaneous urticaria (CSU)
New relief for the intractable itch of chronic hives, or chronic spontaneous urticaria (CSU)
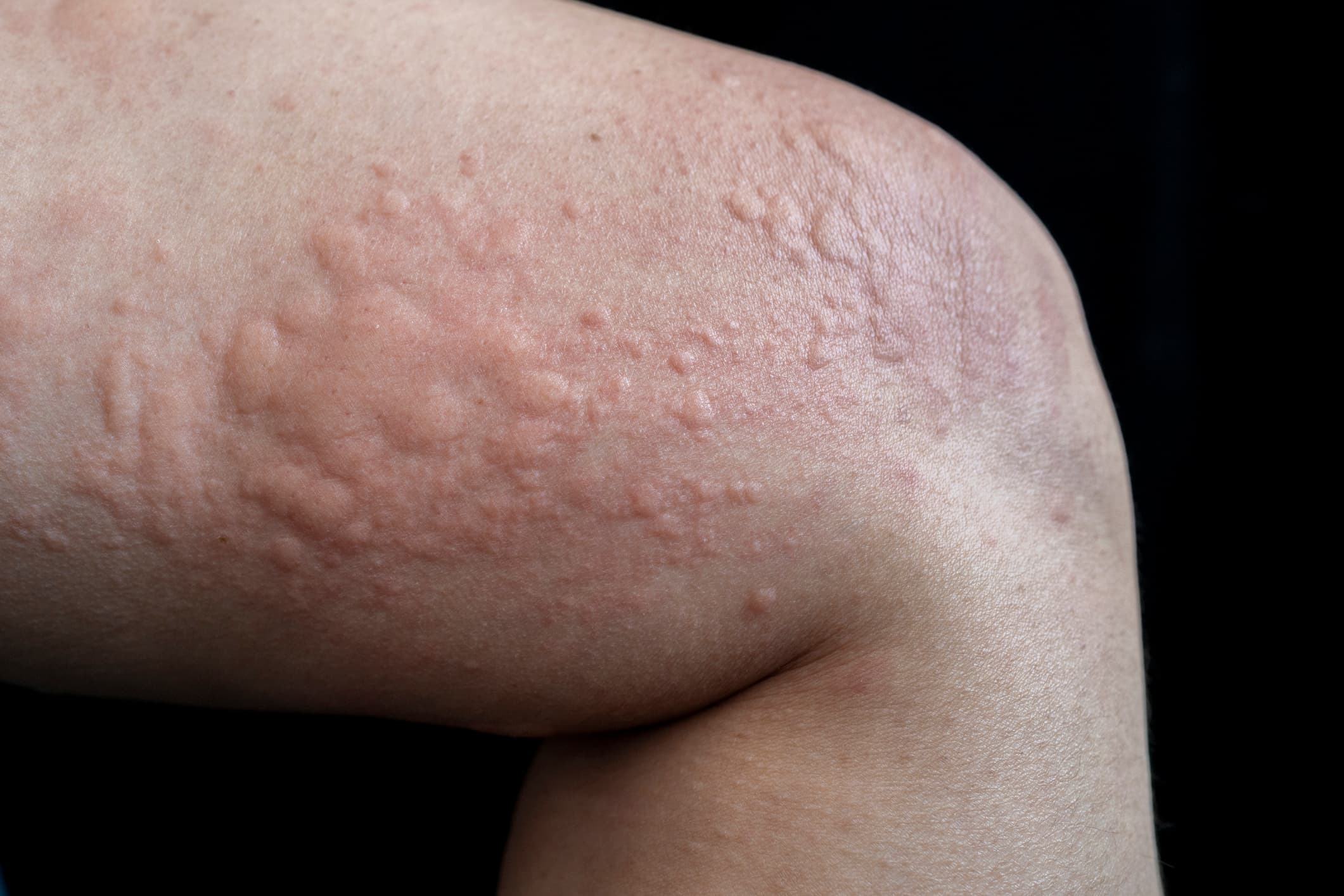 Chronic hives, also known as chronic spontaneous urticaria (CSU), is characterized by persistent hives lasting six weeks or more. It is increasingly understood as an autoimmune condition. In many cases, the immune system mistakenly targets healthy tissue, triggering the release of histamine and other inflammatory chemicals. This leads to the itching, swelling, and redness typical of the condition.
Chronic hives, also known as chronic spontaneous urticaria (CSU), is characterized by persistent hives lasting six weeks or more. It is increasingly understood as an autoimmune condition. In many cases, the immune system mistakenly targets healthy tissue, triggering the release of histamine and other inflammatory chemicals. This leads to the itching, swelling, and redness typical of the condition.
For those who don’t respond to antihistamines, there are now two FDA-approved advanced therapies for CSU: Xolair (omalizumab), a biologic therapy that targets immunoglobulin E (IgE), and Dupixent (dupilumab), another biologic treatment which blocks key inflammatory pathways involved in the immune response.
XOLAIR for chronic hives
XOLAIR (omalizumab) is a once-a-month medical injection for people 12 and older who continue to have hives despite using allergy medications like antihistamines. This treatment is a monoclonal antibody, a lab-made protein that works like a human antibody in the immune system. It helps by targeting IgE (immunoglobulin E), a substance in the body that plays an important role in triggering the release of histamines, responsible for inflammation like hives and CSU.
Dupixent, now also for chronic hives
 Dupixent (dupilumab), already a cornerstone therapy for moderate‑to‑severe atopic dermatitis since 2017, recently earned FDA approval for chronic spontaneous urticaria (CSU) in patients aged 12 and up who remain symptomatic despite antihistamines. The CSU FDA approval for Dupixent stems from results of 3 large clinical trials, Studies A, B, C. Study A and Study C enrolled respectively 136 and 148 chronic hives patients who had never been treated with a biologic drug. The research showed that dupilumab plus antihistamines significantly reduced itch and hives by week 24 versus antihistamines alone. Patients experienced significant improvement in their itch as early as week 3. Study B involved 108 patients whose chronic hives hadn’t responded well to standard antihistamines or who couldn’t tolerate omalizumab treatment. Study B’s findings did not show significant side effects in the patient group which reinforced the safety profile of Dupixent.
Dupixent (dupilumab), already a cornerstone therapy for moderate‑to‑severe atopic dermatitis since 2017, recently earned FDA approval for chronic spontaneous urticaria (CSU) in patients aged 12 and up who remain symptomatic despite antihistamines. The CSU FDA approval for Dupixent stems from results of 3 large clinical trials, Studies A, B, C. Study A and Study C enrolled respectively 136 and 148 chronic hives patients who had never been treated with a biologic drug. The research showed that dupilumab plus antihistamines significantly reduced itch and hives by week 24 versus antihistamines alone. Patients experienced significant improvement in their itch as early as week 3. Study B involved 108 patients whose chronic hives hadn’t responded well to standard antihistamines or who couldn’t tolerate omalizumab treatment. Study B’s findings did not show significant side effects in the patient group which reinforced the safety profile of Dupixent.
Across CSU trials, safety mirrored that in atopic dermatitis and asthma: most common adverse events were injection-site reactions; no new safety signals emerged.
If you suffer from chronic hives, a life-altering skin disease, find out what solutions are now possible by consulting with our skilled dermatologists at SkinCare Physicians.



Leave a Reply